
The Telegraph recently reported that the public has been told to stop taking cough and cold medicines over safety fears concerning the drug pholcodine, an opioid cough suppressant.
Twenty of the common cough and cold medicines, including Day and Night Nurse capsules, have been urgently withdrawn from the market on the order of the drug regulators because of concerns about a “very rare” risk of anaphylaxis, a life-threatening adverse event.
When it comes to the mRNA COVID-19 vaccines, the regulatory double standards have never been so glaringly obvious.
Anaphylaxis was identified as an important risk by the European Medicines Agency, as early as December 2020, in the EMA’s CHMP (Committee for Medicinal Products for Human Use) assessment report on the Pfizer-BioNTech COVID-19 vaccine, seen below.
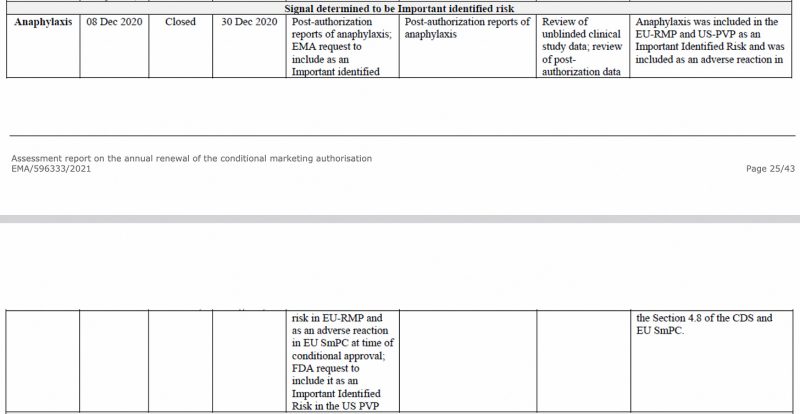
Also, in the EU’s first Periodic Safety Update Report, which I have previously analysed, anaphylaxis was again flagged as an important identified risk.

In this pharmacovigilance report, 3,827 relevant cases (individuals) were identified from the post-authorization data. The country with the highest incidence was Japan, followed by the US and the UK.
The highest number of cases reported were among women, a shocking 3,182 cases compared to 454 cases for men, with a median age of 44. The fact that 7 times more cases were reported for women is nothing new. Back in December 2021, I analysed the Pfizer-prepared document for the FDA, covering the 3-month period, Dec 2020 through Feb 28, 2021 – in the case of anaphylaxis- women were 8 times more affected.
So, 98 percent of the relevant adverse events (including anaphylactic reaction, anaphylactic shock, anaphylactoid reaction and anaphylactoid shock) were classified as serious!
Furthermore, for 92 percent of the events, the time elapsed for an adverse event to occur after vaccine administration was less than 24 hours.

Fatal outcomes
Of the 3,922 events, 28 were fatal, and for a staggering 704, the outcome was unknown. No case numbers were given for fatal outcomes.

Cases by age group
Of the 3,827 relevant cases (individuals), 23 were from the pediatric age group and 3,021 were from the adult age group.
Presence of comorbidities
What’s noteworthy is that roughly 2/3 of all anaphylaxis cases did not have any comorbidities (underlying health issues).

Given what has transpired since the mRNA COVID-19 vaccines have been rolled out, it comes as no surprise to read: “no new safety information was identified pertaining to the risk of anaphylaxis with BNT162b2” (Pfizer-BioNTech COVID-19 vaccine). The reason given (or the excuse they hide behind) is that ‘this risk is communicated . . . which includes information on appropriate action to be taken, as follows: “As with all injectable vaccines, appropriate medical treatment and supervision must always be readily available in case of a rare anaphylactic event following the administration of the vaccine.”
Under Regulation 174, Information for UK healthcare Professionals, which was last revised in Dec 2021, the following is stated:

What’s more, Pfizer/BioNTech’s lipid nanoparticle ingredients ALC-0159 and ALC-0315 have never been included in any licensed drug before. ALC-0159 contains PEG (Polyethylene glycol), which is known to cause anaphylaxis.
It’s unequivocal: anaphylaxis was a known life-threatening adverse event around the same time emergency use authorization was granted for the Pfizer-BioNTech COVID-19 vaccine. Yet, because it’s an “injectable vaccine,” it somehow has gotten a free pass from all the drug regulators, no matter how much damning data accumulates when a cough syrup or capsule, on the other hand, gets urgently recalled on the basis of “a very rare risk of anaphylaxis.”
Republished from Brownstone Institute.

